Purpose of LUNIRI

PD Dr. med. Steffen Frese, founder of the non-profit organization LUNIRI.
LUNIRI was founded by PD Dr. med. Steffen Frese (Figure 1). It is a non-profit organization registered at the district court Berlin-Charlottenburg (reference number HRB 161256 B). The state Berlin accredited LUNIRI as a non-profit organization on 24th Sept 2014 (tax reference number 27/604/02643).
The name LUNIRI is composed of the terms lupus nephritis and irinotecan. This name stands for the purpose of the non-profit organization: to acquire donations which will help establish a potentially new treatment option for the autoimmune disease systemic lupus erythematosus. In this way, LUNIRI will support both clinical and basic research projects. To ensure the appropriate use of the donations, LUNIRI is only allowed to transfer research support to university research organizations and not to private or pharmaceutical facilities.
At present, the initiation of a clinical study using irinotecan in patients with SLE will be given the first priority. We plan a first clinical trial which will recruit patients with refractory lupus nephritis. These patients suffer from active lupus nephritis despite taking conventional lupus medication, thus have the highest need for new therapeutic approaches.
At the beginning of the planned clinical trial patients will be treated with low-dose irinotecan parallel to the standard SLE medication. Providing the existing lupus nephritis stabilizes or improves, concomitant standard SLE medication will be reduced (Figure 2). The trial is planned as a multi-center study; so far three university centers in Germany have agreed to participate in the study.
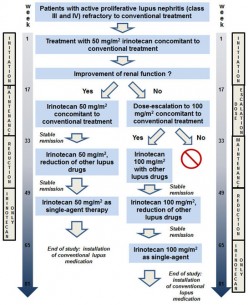 Scheme of the study protocol for a first clinical trial with SLE and irinotecan called LUNIRI-ct1. Patients with refractory lupus nephritis will be recruited for the study. At the beginning the treatment will be performed with low-dose irinotecan concomitant with SLE standard medication. Providing there is an improvement in or stabilization of renal function, a reduction in standard medication is planned during the later course of the trial.
Scheme of the study protocol for a first clinical trial with SLE and irinotecan called LUNIRI-ct1. Patients with refractory lupus nephritis will be recruited for the study. At the beginning the treatment will be performed with low-dose irinotecan concomitant with SLE standard medication. Providing there is an improvement in or stabilization of renal function, a reduction in standard medication is planned during the later course of the trial.If you have systemic lupus erythematosus yourself or if you have relatives or friends afflicted with the disease – please donate to LUNIRI and give these patients the chance to receive a new therapeutic option.

