SLE and Irinotecan
I am a surgeon and since 2014 I have specialized in thoracic surgery. As an academic surgeon, I have been a research group leader for many years. The initial interest of my work was cancer research. As a surgeon, I was not really aware of systemic lupus erythematosus (SLE) or other autoimmune diseases until an experiment about seven years ago which started as a routine cancer research experiment. The results of this experiment were so unexpected that I mused about them more than half a year before deciding to follow up the case. The hypothesis was that it might possible to suppress fatal hypersensitivity reaction with the topoisomerase I inhibitor irinotecan. To evaluate this hypothesis we started with anaphylactic shock and failed. We continued with autoimmune diabetes and failed again. This situation evoked serious doubts about this unusual project, which – at that time – was not supported by third-party funds. I began to think that it might have been a pipe dream. However, a couple of weeks later our lupus-prone mice suddenly demonstrated amazing effects.
The mice used for these experiments were the first generation of mice crossbred from New Zealand Black and New Zealand White mice (two natural occurring strains), known as NZB/NZW mice. NZB/NZW mice spontaneously develop a lupus-like disease with antinuclear antibodies, hemolytic anemia, proteinuria, and progressive glomerulonephritis resembling human SLE. [1] However, although this animal model has been used for more than 60 years and more than 700 publications with NZB/W mice exist, only very few research groups have been able to successfully treat NZB/W mice with advanced (or established) lupus nephritis which most likely reflects the patient’s situation. Therapy of established lupus nephritis means that treatment started at the point when the mice already had damaged kidneys, demonstrated by a loss of blood protein. The loss of blood protein can be measured by increased levels of protein in the urine (proteinuria). Interestingly, with the current standard SLE medication it was not possible to reverse established proteinuria in NZB/W mice.[2] [3] [4]
We were able to show that the topoisomerase I inhibitor irinotecan improved advanced lupus nephritis and significantly prolonged the survival of NZB/W mice.[5] However, an unfavorable aspect of these first findings was that we had applied doses of irinotecan similar to those used in chemotherapy to treat humans. Hence it was questionable whether irinotecan might be a realistic new treatment option for a disease which mainly affects women of childbearing age. Consequently, we started experiments evaluating the effect of reduced irintecan doses on the course of SLE. Figure 1 shows such an experiment in lupus-prone mice when an irinotecan dose was applied which was eight times lower than the dose used for chemotherapy. Finally, we were able to demonstrate that an irinotecan dose which was 50 times lower than the dose used for chemotherapy ameliorated existing lupus nephritis and significantly prolonged survival in lupus-prone NZB/W mice.[6]
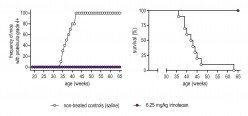 Figure 1: Suppression of lupus nephritis and prolonged survival of lupus-prone NZB/W mice treated with low-dose irinotecan. The onset of lupus nephritis was determined by the loss of protein in the urine (proteinuria). A proteinuria grade 4+ corresponds to the level of severe lupus nephritis (published in Arthritis Rheumatol. 2014 Aug; 66(8):2259-69).
Figure 1: Suppression of lupus nephritis and prolonged survival of lupus-prone NZB/W mice treated with low-dose irinotecan. The onset of lupus nephritis was determined by the loss of protein in the urine (proteinuria). A proteinuria grade 4+ corresponds to the level of severe lupus nephritis (published in Arthritis Rheumatol. 2014 Aug; 66(8):2259-69).Based on these results, we deduced that the effects and mechanisms of irinotecan used in chemotherapy are not the same those used for the suppression of SLE. This hypothesis was supported by subsequent laboratory investigations aimed at discovering how the molecular mechanism of irinotecan influenced the course of SLE. So far the following results have been obtained:
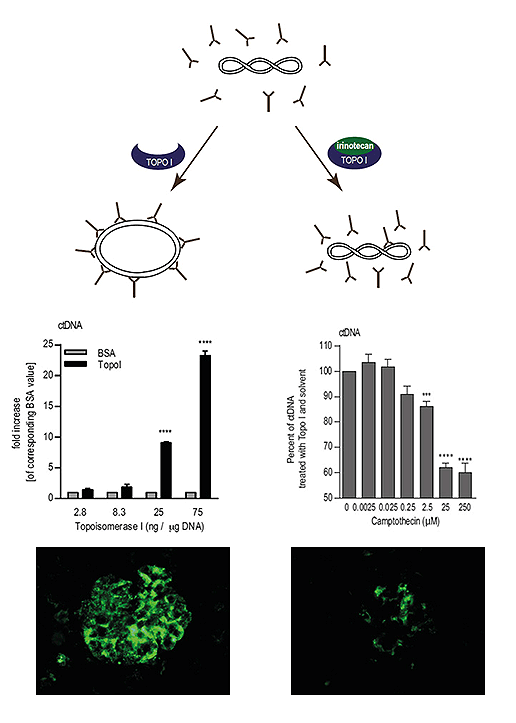 Figure 2: Scheme illustrating how the level of DNA relaxation influences the level of lupus-derived auto-antibody binding directed against double-stranded DNA. It is further shown how this effect might explain irinotecan-mediated effects on lupus nephritis (published in J Immunol. 2010 Feb 15; 184(4):2175-82 and Arthritis Rheumatol. 2014 Aug; 66(8):2259-69).
Figure 2: Scheme illustrating how the level of DNA relaxation influences the level of lupus-derived auto-antibody binding directed against double-stranded DNA. It is further shown how this effect might explain irinotecan-mediated effects on lupus nephritis (published in J Immunol. 2010 Feb 15; 184(4):2175-82 and Arthritis Rheumatol. 2014 Aug; 66(8):2259-69).- In contrast to conventional lupus drugs irinotecan induced only a mild and transient suppression of the immune system.[5], [6]
- Irinotecan prevented the generation of programmed cell death (apoptosis) in the kidneys of lupus-prone mice.[5] This effect contradicts the use of irinotecan as a chemotherapeutic agent where irinotecan is applied for the induction of apoptosis.
- Inhibition of topoisomerase I influenced the degree of DNA relaxation, and the level of DNA relaxation affected the binding of pathogenic lupus-derived auto-antibodies directed against double-stranded DNA. This effect was not in line with our initial hypothesis which suggested that the production of single-stranded DNA breaks is responsible for irinotecan-mediated effects on lupus nephritis.[7] Again by chance we found that topoisomerase I alone increased the binding of lupus-typical anti-DNA antibodies (Figure 2, left panel). Enhanced anti-DNA binding was reversed by inhibition of topoisomerase I (Figure 2, right panel)[6] , an effect which has been shown only in vitro so far. Whether this effect is also responsible for the irinotecan-mediated reduction of detrimental immune deposits found in the kidneys of lupus-prone mice (Figure 2, lower panel) is still unclear, and a subject of future research projects..
- ↑ Hahn, B.H. Lessons in lupus: the mighty mouse. Lupus 10, 589-593 (2001).
- ↑ Casey, T.P. Immunosuppression by cyclophosphamide in NZB X NZW mice with lupus nephritis. Blood 32, 436-444 (1968).
- ↑ Casey, T.P. Azathioprine administration to NZB X NZW hybrid mice with lupus nephritis: beneficial effect complicated by development of malignant lymphomas. N Z Med J 79, 290-295 (1973).
- ↑ Steinberg, A.D., Gelfand, M.C., Hardin, J.A. & Lowenthal, D.T. Therapeutic studies in NZB/W mice. III. Relationship between renal status and efficacy of immunosuppressive drug therapy. Arthritis Rheum 18, 9-14 (1975).
- ↑ Frese-Schaper, M., Zbaeren, J., Gugger, M., Monestier, M. & Frese, S. Reversal of established lupus nephritis and prolonged survival of New Zealand black x New Zealand white mice treated with the topoisomerase I inhibitor irinotecan. J Immunol 184, 2175-2182 (2010).
- ↑ Frese-Schaper, M., et al. Low-dose irinotecan improves advanced lupus nephritis in mice potentially by changing DNA relaxation and anti-double-stranded DNA binding. Arthritis Rheumatol 66, 2259-2269 (2014).
- ↑ Frese, S. & Diamond, B. Structural modification of DNA-a therapeutic option in SLE? Nat Rev Rheumatol 7, 733-738 (2011).

