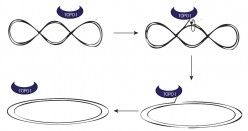
Topoisomerase I and Irinotecan
Topoisomerase I is an enzyme (i.e. a protein catalyzing certain biochemical reactions) which is expressed in almost every cell of the body and which is highly conserved in the phylogenetic tree.[1] Its major function is the relaxation of DNA (desoxyribonucleic acid, the genetic material). Most DNA exists as double helix (i.e. double-stranded). The double helix is normally self-winding to achieve compact DNA which fits into the small nucleus of the cell. Under certain circumstances such as cell division an over-winding (supercoiling) of the DNA takes place. Such supercoiling of the double-stranded DNA induces torsional stress at the DNA helix bearing the risk of uncontrolled strand breaks. In order to release torsional stress from the DNA, topoisomerase I binds to the DNA and cleaves one DNA strand. This allows the rotation of the cleaved strand around the other in a controlled reaction (DNA relaxation). Afterwards, the nicked strand is re-ligated by topoisomerase I, thus restoring intact double-stranded DNA. (Figure 1) [2]
Topoisomerase I inhibitors stabilize a normally very transient catalytic intermediate in which topoisomerase I is bound to one strand of the DNA, known as the topoisomerase I cleavable complex. When the cleavable complex is stalled by topoisomerase I inhibitors, re-ligation of the DNA is impossible (Figure 2, left panel).[3]
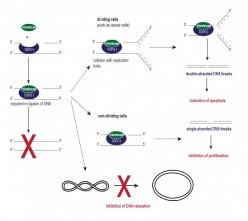 Figure 2: Effects of the topoisomerase I inhibitor irinotecan on the structure of DNA with subsequent cellular changes.
Figure 2: Effects of the topoisomerase I inhibitor irinotecan on the structure of DNA with subsequent cellular changes.The consequences of prevented re-ligation vary; in proliferating cells the stalled topoisomerase I cleavable complex may collide with replication forks resulting in unrepairable DNA double strand breaks followed by cell death also known as apoptosis (Figure 2, right upper panel).[4], [5] For this reason, topoisomerase I inhibitors are used as anticancer drugs to treat several types of tumors. [6] In non-dividing cells the treatment with topoisomerase I inhibitors results in the production of single-stranded DNA breaks (Figure 2, right middle panel). Single-stranded DNA breaks are believed to reduce a cell’s replication capacity, which is not lethal.[7] For a long time we thought that the induction of single-stranded DNA breaks might explain the effects of irinotecan on lupus nephritis. Considering the detrimental role of lupus-typical auto-antibodies against double-stranded DNA, we hypothesized that the postulated induction of DNA single strand breaks might modify the DNA in a way that impairs the binding of anti-double-stranded DNA antibodies to the DNA.[8] Due to our hypothesis, we paid much less attention to a third effect of topoisomerase I inhibitors, that of inhibited DNA relaxation. Normally topoisomerase I binds to the DNA, induces DNA relaxation, re-ligates the DNA and moves along the DNA to the next supercoiled site. However, if a topoisomerase I inhibitor stabilizes the cleavable complex, topoisomerase I is no longer able to move along the DNA and the process of DNA relaxation is interrupted (Figure 2, right lower panel).[10], [11]
- ↑ Koiwai, O. et al. Cloning of the mouse cDNA encoding DNA topoisomerase I and chromosomal location of the gene. Gene 125, 211-6 (1993).
- ↑ Pommier, Y. Topoisomerase I inhibitors: camptothecins and beyond. Nat Rev Cancer 6, 789-802 (2006).
- ↑ Hertzberg, R.P., Caranfa, M.J. & Hecht, S.M. On the mechanism of topoisomerase I inhibition by camptothecin: evidence for binding to an enzyme-DNA complex. Biochemistry 28, 4629-38 (1989).
- ↑ Bjornsti, M.A., Benedetti, P., Viglianti, G.A. & Wang, J.C. Expression of human DNA topoisomerase I in yeast cells lacking yeast DNA topoisomerase I: restoration of sensitivity of the cells to the antitumor drug camptothecin. Cancer Res 49, 6318-23 (1989).
- ↑ Hsiang, Y.H., Lihou, M.G. & Liu, L.F. Arrest of replication forks by drug-stabilized topoisomerase I-DNA cleavable complexes as a mechanism of cell killing by camptothecin. Cancer Res 49, 5077-82 (1989).
- ↑ Rothenberg, M.L. Efficacy and toxicity of irinotecan in patients with colorectal cancer. Semin Oncol 25, 39-46 (1998).
- ↑ Hsiang, Y.H., Hertzberg, R., Hecht, S. & Liu, L.F. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem 260, 14873-8 (1985).
- ↑ Frese, S. & Diamond, B. Structural modification of DNA-a therapeutic option in SLE? Nat Rev Rheumatol 7, 733-8 (2011).
- ↑ Champoux, J.J. DNA topoisomerases: structure, function, and mechanism. Annu Rev Biochem 70, 369-413 (2001).
- ↑ Stewart, L., Ireton, G.C. & Champoux, J.J. A functional linker in human topoisomerase I is required for maximum sensitivity to camptothecin in a DNA relaxation assay. J Biol Chem 274, 32950-60 (1999).
- ↑ Lisby, M. et al. Residues within the N-terminal domain of human topoisomerase I play a direct role in relaxation. J Biol Chem 276, 20220-7 (2001).